Yuki YAMADA
Position: Professor
Degree: Ph.D.
Laboratory: Energy Materials Science
to Laboratory Homepage
Development of New Battery Materials and Reactions
Rechargeable batteries can store electrical energy as chemical energy and discharge it when needed. We are developing new materials and reactions toward high-energy-density and safe rechargeable batteries. For example, through our unique strategy of controlling the coordination states of ions and solvent molecules in liquid electrolyte materials, we are developing new functions and properties that are not shared with conventional materials. So far, we have discovered unusual reduction stability, high oxidation stability above 5 V, faster electrode reactions, suppressed metal corrosion, and flame retardance, etc. These unusual functions will enable higher voltage operation, faster charging, and higher safety of lithium-ion batteries. We will continue to develop new materials and reactions through our unique strategies. 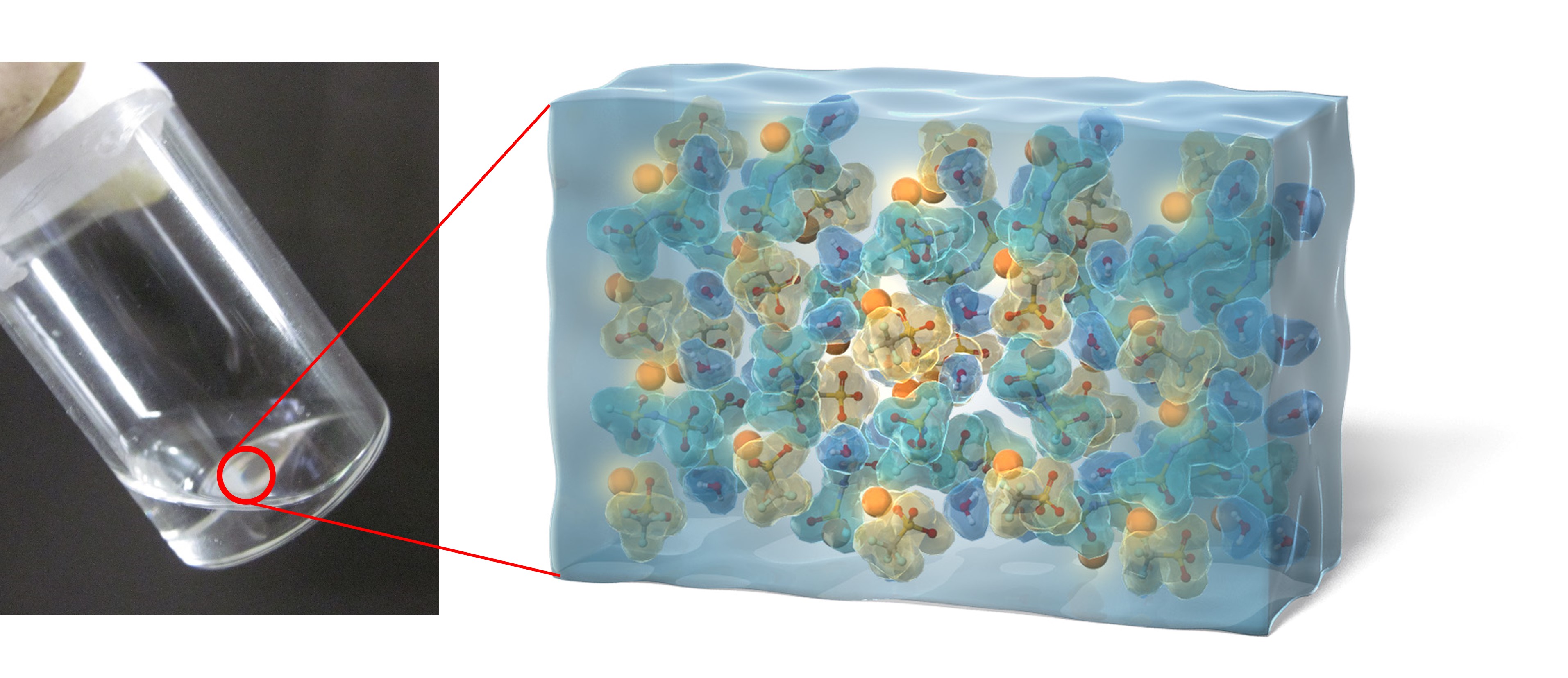
Development of New Rechargeable Batteries
Lithium-ion batteries, used in smartphones, notebook computers, and other portable devices, are indispensable in our daily lives. Recently, with an increasing demand for electric vehicles, there is a need to develop new rechargeable batteries with higher energy density and higher safety. We are working on the development and commercial application of new rechargeable batteries by utilizing the unique functions and properties of our original electrolyte materials. One such device is a high-voltage aqueous battery (a battery that uses water as the electrolyte solvent). Conventional aqueous batteries (nickel-metal hydrate batteries, lead-acid batteries, etc.) have a lower risk of fire, but their operation voltage is lower than 2 V due to the electrolysis of water. In contrast, we have proposed a 3 V-class aqueous battery by utilizing a new electrolyte material, a room-temperature hydrate melt, which has a significantly high voltage stability of >3 V. This new aqueous battery has a minimized risk of fire owing to the nonflammable aqueous electrolyte and delivers a high voltage of over 3 V, which is comparable to the conventional lithium-ion batteries (2.4~3.8 V). We will continue to challenge the limits of aqueous batteries and develop other next-generation batteries that have yet to be realized.
Establishment of New Theories in Electrochemistry
“Electrochemistry” deals with the interconversion of electrical and chemical energy, which is the essence of battery reactions. Conventional lithium-ion batteries and fuel cells are designed based on the theories in electrochemistry. However, the unusual functions and properties we have discovered in our new electrolyte materials cannot be understood based on conventional electrochemistry. This is because electrochemistry was originally developed for extremely dilute electrolytes (with low salt concentration) where ion-ion interactions are negligible. We are developing new theories of electrochemistry that can be applied to the new electrolyte materials. Specifically, through detailed analysis of ion-ion interactions, ion-solvent interactions, and electrode/electrolyte interfaces, we will reconsider the theories and interfacial structures and establish new electrochemistry that will be published in future textbooks. 
Message to Students
We are a new laboratory established in April 2021. We welcome those who want to approach energy issues based on “chemistry” and those who are interested in next-generation rechargeable batteries that can safely store large amounts of energy.
Publication
- Qifeng Zheng, Yuki Yamada, Rui Shang, Seongjae Ko, Yun-Yang Lee, Kijae Kim, Eiichi Nakamura, Atsuo Yamada, A cyclic phosphate-based battery electrolyte for high voltage and safe operation, Nature Energy, 5, 291-298 (2020). https://doi.org/10.1038/s41560-020-0567-z
- Yuki Yamada, Jianhui Wang (co-first author), Seongjae Ko, Eriko Watanabe, Atsuo Yamada, Advances and issues in developing salt-concentrated battery electrolytes, Nature Energy, 4, 269-280 (2019). https://doi.org/10.1038/s41560-019-0336-z
- Jianhui Wang, Yuki Yamada, Keitaro Sodeyama, Eriko Watanabe, Koji Takada, Yoshitaka Tateyama, Atsuo Yamada, Fire-extinguishing organic electrolytes for safe batteries, Nature Energy, 3, 22-29 (2018). https://doi.org/10.1038/s41560-017-0033-8
- Yuki Yamada, Kenji Usui, Keitaro Sodeyama, Seongjae Ko, Yoshitaka Tateyama, Atsuo Yamada, Hydrate-melt electrolytes for high-energy-density aqueous batteries, Nature Energy, 1, Article number:16129 (2016). https://doi.org/10.1038/NENERGY.2016.129
- Jianhui Wang, Yuki Yamada (co-first author), Keitaro Sodeyama, Ching Hua Chiang, Yoshitaka Tateyama, Atsuo Yamada, Superconcentrated electrolytes for a high-voltage lithium-ion battery, Nature Communications, 7, Article number:12032 (2016). https://doi.org/10.1038/ncomms12032
- Yuki Yamada, Keizo Furukawa, Keitaro Sodeyama, Keisuke Kikuchi, Makoto Yaegashi, Yoshitaka Tateyama, Atsuo Yamada, Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries, Journal of the American Chemical Society, 136, 5039-5046 (2014). https://doi.org/10.1021/ja412807w
