Yasuyuki KONDO
 Position: Assistant professor
Position: Assistant professor
Degree: Ph.D.
Laboratory: Energy Materials Science
to Laboratory Homepage
Analysis of Charge-Discharge Reaction Mechanism in Lithium-Ion Batteries
Recently, electric vehicles (EVs) utilizing lithium-ion batteries have been commercialized. To develop higher-performance EVs, rapid charge-discharge is strongly required for the batteries. Charge-discharge reactions of lithium-ion batteries can be simply explained as the reversible shuttle of lithium-ion between positive and negative electrodes. However, internal resistances in lithium-ion batteries are actually derived from several reaction steps. Hence, there is a large demand to understand the charge-discharge reaction mechanism in lithium-ion batteries for reducing the internal resistances. Our group aims to fundamentally analyze the origin of the internal resistances and the reaction mechanism in lithium-ion batteries. It is well known that the energy barrier in an interfacial lithium-ion transfer at solid/liquid interface between electrodes and electrolyte solutions is significantly large in most of the lithium-ion batteries, which is the large obstacle for the rapid charge-discharge. We clarified that the energy barriers of interfacial lithium-ion or sodium-ion at electrode/electrolyte interfaces depend on the electrolyte compositions. We will elucidate the reaction mechanisms of various rechargeable batteries, propose a clear guideline for accelerating the reactions, and develop next-generation energy-storage devices with rapid charge-discharge rate.
Development of Next-Generation Energy-Storage Device Technology
Lithium-ion batteries consist of layered transition metal oxides as positive electrodes, graphite as negative electrodes and organic electrolyte solutions as lithium-ion conductive electrolytes. Because their energy density has already approached the theoretical value, pioneering novel electrode materials and/or battery reactions is desired for developing next-generation batteries with higher energy density. Nevertheless, some conventional electrode materials can also be charged and discharged with other ion species, which may lead to the development of new battery reactions. For example, graphite can accommodate various cations and even anions in its interlayer spaces. Such reactions may be applied to negative and positive electrodes for high-energy-density batteries. As for battery safety, development of non-flammable electrolytes is necessary to minimize the risk of battery fires especially for large-scale applications such as EVs. Our group aims to develop next-generation energy-storage devices with high energy density and high safety. For example, we have achieved the reversible anion intercalation into/from graphite in neutral aqueous solutions. Hence, graphite can be used as a positive electrode for aqueous energy-storage devices with high safety. Our group will develop the high-performance energy-storage devices by exploring such novel battery materials and/or reactions. 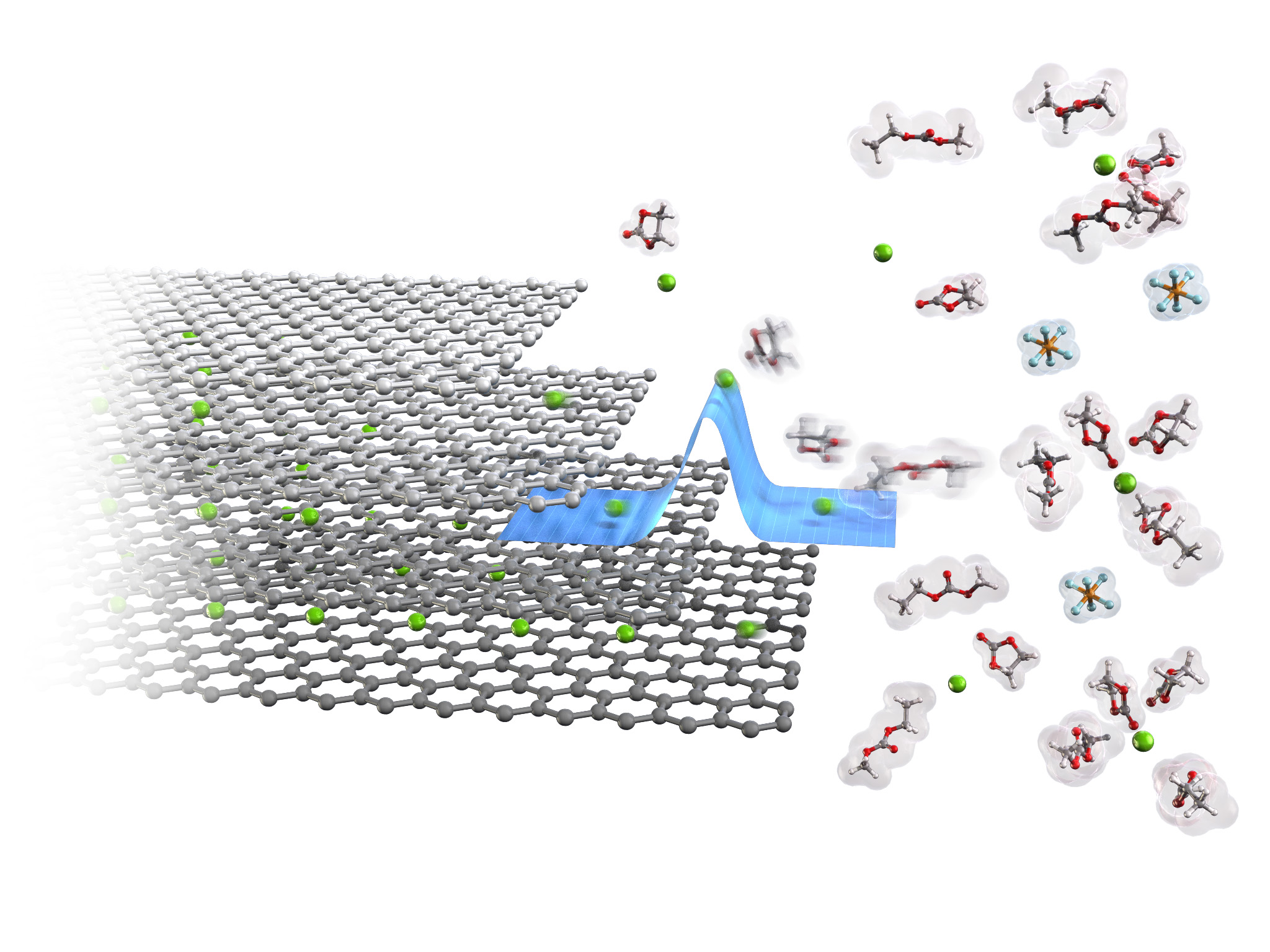
Publication
- Y. Kondo, T. Fukutsuka, K. Miyazaki, Y. Miyahara, and T. Abe: “Investigation of Electrochemical Sodium-Ion Intercalation Behavior into Graphite–Based Electrodes” J. Electrochem. Soc. 166 (3) A5325–A5327 (2019).
- Y. Kondo, Y. Miyahara, T. Fukutsuka, K. Miyazaki, and T. Abe: “Electrochemical Intercalation of Bis(fluorosulfonyl)amide Anions into Graphite from Aqueous Solutions” Electrochem. Commun. 100 26–29 (2019).
- Y. Kondo, T. Fukutsuka, Y. Yokoyama, Y. Miyahara, K. Miyazaki, and T. Abe: “Kinetic Properties of Sodium–Ion Transfer at the Interface between Graphitic Materials and Organic Electrolyte Solutions” J. Appl. Electrochem. 51 (4) 629–638 (2021).