Hisanao HAZAMA
Position: Associate Professor
Degree: Doctor of Engineering
Laboratory: Medical Beam Physics
to Laboratory Homepage
Development of Safe and Minimally Invasive Optical Diagnostic and Therapeutic Technologies
We are engaged in the development of devices and methodologies that enable the safe diagnosis and treatment of a wide range of diseases using light from lasers and light-emitting diodes (LEDs). Targeted conditions include cancer, dental caries (tooth decay), atherosclerosis, benign prostatic hyperplasia, varicose veins of the lower extremities, and urinary stones. A distinctive feature of our work is our close collaboration not only with engineers, but also with physicians, dentists, and medical device manufacturers, with the aim of achieving clinical implementation.
Recently, we have been approaching practical application of a system that delivers laser light into the body using endoscopes and optical fibers, enabling the safe treatment of early-stage gastrointestinal cancers (esophageal, gastric, and colorectal cancers). In addition, a device that allows simple measurement of tooth hardness for accurate diagnosis of dental caries has already been commercialized.
In parallel with experimentally verifying the effectiveness of new therapeutic approaches, we are also developing computational simulation models to accurately predict treatment outcomes. Through these efforts, we are establishing a foundation for the rapid clinical translation of new treatment modalities.
Photodynamic therapy (PDT), which is already used clinically, is a minimally invasive cancer treatment in which photosensitizing agents that selectively accumulate in cancer cells are administered, followed by light irradiation to generate reactive oxygen species that selectively destroy cancer cells. To expand the range of cancers to which PDT can be applied and to optimize treatment conditions for improved therapeutic efficacy, we actively employ computational simulations.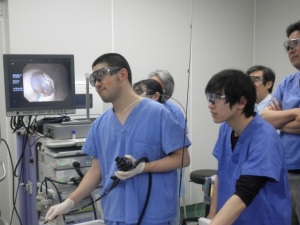
Development of Laser Ionization Mass Spectrometry Technologies
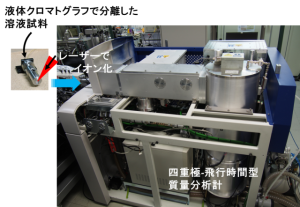
Laser-based ionization enables the ionization of very large molecules with molecular weights exceeding several tens of thousands without causing fragmentation. By performing mass spectrometric analysis of these ions, it is possible to analyze biologically and medically important molecules such as proteins and pharmaceuticals. Because biological samples contain a vast number of coexisting substances, mass spectrometry is commonly performed after separation by liquid chromatography. However, conventional laser ionization is typically carried out under vacuum conditions, which makes direct coupling with liquid chromatography difficult.
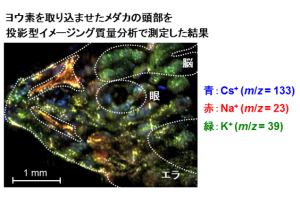
To address this limitation, we are developing instrumentation that enables high-speed analysis of biological samples by irradiating sample solutions with an infrared laser under atmospheric pressure to induce ionization, and by directly coupling this process to mass spectrometry. In addition, we are also conducting research on mass spectrometry imaging, a technique that combines mass spectrometric analysis with imaging. This approach allows simultaneous measurement of the spatial distributions of individual substances based on their mass-to-charge ratios.
By employing atmospheric-pressure ionization using infrared lasers, as described above, we are developing a high-resolution mass spectrometry imaging system capable of achieving cellular-level spatial resolution that is not attainable with commercially available instruments.
Publications
- Riku Hirotani, Yuto Miyoshi, Varun Sendilraj, and Hisanao Hazama: “Atmospheric pressure mass spectrometry imaging using electrospray-assisted laser desorption/ionization with gas
transportation through a heated tube and minimal sample preparation,” Mass Spectrometry 13(1), A0167 (2024). - Sochi J. Ogbonna, Katsuyoshi Masuda, and Hisanao Hazama: “The effect of fluence rate and wavelength on the formation of protoporphyrin IX photoproducts,” Photochemical & Photobiological Sciences 23(9), 1627–1639 (2024).
- Sochi J. Ogbonna, William Y. York, Takahiro Nishimura, Hisanao Hazama, Hideo Fukuhara, Keiji Inoue, and Kunio Awazu: “Increased fluorescence observation intensity during the photodynamic diagnosis of deeply located tumors by fluorescence photoswitching of protoporphyrin IX,” Journal of Biomedical Optics 28(5), 055001 (2023).
- Sota Kondo, Hisanao Hazama, Yutaka Tomioka, Atsushi Mine, Satoshi Yamaguchi, Saeko Okumura, Hiroaki Tanimoto, Kenzo Yasuo, Kazushi Yoshikawa, Kazuyo Yamamoto, and Kunio Awazu: “Demonstration of an optical dentin hardness measuring device using bovine dentin with different demineralization times,” Journal of Biomedical Optics, 27(10), 105004 (2022).
- Aya Kasakawa, Shinichi Sekine, Kenji Tanaka, Jumpei Murakami, Sota Kondo, Hisanao Hazama, Kunio Awazu, and Shigehisa Akiyama: “Effect of Q-switched Er:YAG laser irradiation on bonding performance to dentin surface,” Dental Materials Journal 41(4), 616–623 (2022).
- Hiroki Kannen, Yuto Miyoshi, Hisanao Hazama, and Kunio Awazu: “Improvement in ionization efficiency using metal oxide nanoparticles in laser desorption/ionization mass spectrometry of a cancer drug,” Mass Spectrometry 10(1), A0099, 1–5 (2021).
- Sochi Judith Ogbonna, Hisanao Hazama, and Kunio Awazu: “Mass spectrometric analysis of the photobleaching of protoporphyrin IX used in photodynamic diagnosis and therapy of cancer,” Photochemistry and Photobiology 97(5), 1089–1096 (2021).
- Yu Shimojo, Takahiro Nishimura, Hisanao Hazama, Nobuhisa Ito, and Kunio Awazu: “Incident fluence analysis for 755-nm picosecond Laser treatment of pigmented skin lesions based on threshold fluences for melanosome disruption,” Lasers in Surgery and Medicine 53(8), 1096–1104 (2021).
- Sharmin Akter, Sachiko Saito, Mizuho Inai, Norihiro Honda, Hisanao Hazama, Tomoyuki Nishikawa, Yasufumi Kaneda, and Kunio Awazu: “Efficient photodynamic therapy against drug-resistant prostate cancer using replication-deficient virus particles and talaporfin sodium,” Lasers in Medical Science 36(4), 743–750 (2021).